When passing matter, X-rays are absorbed. If an X-ray beam of the intensity I passes an absorber of the thickness x with a photon energy dependent linear absorption coefficient µ(E), the intensity loss will be in good approximation proportional to the intensity and the absorber thickness:
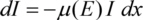
Integrating this expression delivers the part I(x) of the incident intensity I0 found behind the absorber:
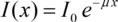
The linear absorption coefficient µ is correlated with the absorption coefficient β via
![]()
where β (listed values e.g.) is the imaginary part of the complex index of refraction n*:
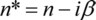
There are three effects involved in X-ray absorption: the Compton-effect, the photoelectric effect and the pair production. Each of them contributes to the absorption cross section σtotal , giving the probability for a photon to interact when passing a certain thickness of matter:
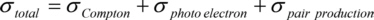
For most X-ray optical applications with photon energies below 1 MeV are used, the greatest part of absorption is photoelectric absorption.
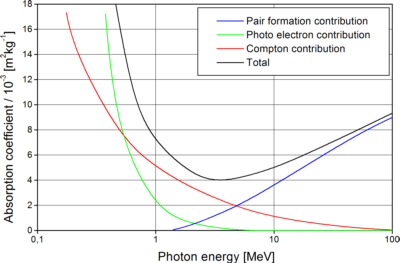
Fig. 1: The total mass absorption coefficient for Pb with contributions of photoelectric absorption, Compton scattering and electron-positron pair formation [Eng 1966]
Photoelectric absorption occurs, when an X-ray photon transfers energy to an electron in the absorber. As electrons bound in an atom show discrete energy levels, there are only certain possible portions of energy to undergo transitions between the different levels. These are shown in fig. 2.
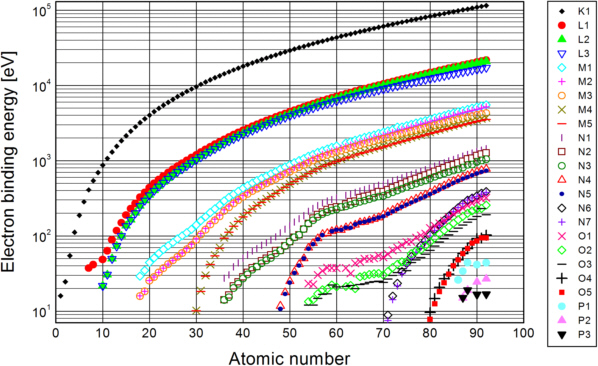
Fig. 2: Relation between electron binding energies, atomic number Z and electronic transition: download the data [Hen 1993]
For the inner levels, the binding energy grows approximately proportional to the square of the atomic number Z. This is known as the Moseley's law (Henry Moseley, 1913):
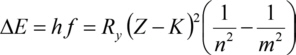
With Planck constanth, the frequency f of the photons absorbed (or emitted) during a transition of an electron between the electron levels with the main quantum numbers m and n, Rydberg constantRy, the atomic number Z and a screening constant K (correction to account for the screening effect of electrons between the considered electron and the nucleus, screening the charge of the nucleus). Ry is
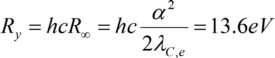
with the fine structure constant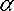 and the Compton wavelengthλC,e of the electron.
and the Compton wavelengthλC,e of the electron.
| [Eng 1966] | H. A. Enge, Introduction to nuclear physics, p. 193, Addison-Wesley, 1966 |
| [Hen 1993] |
B. L. Henke, E. M. Gullikson, and J. C. Davis. X-ray interactions: photoabsorption, scattering, transmission, and reflection at E=50-30000 eV, Z=1-92, Atomic Data and Nuclear Data Tables Vol. 54 (no.2), p. 181-342, July 1993 |
| [Mos 1913] | H. G. J. Moseley, The High Frequency Spectra of the Elements, Phil. Mag., p. 1024, 1913 |


